Abstract
Background: Standard of care for patients with newly diagnosed multiple myeloma (NDMM) who are ≤ 70 years of age and who are fit is induction therapy followed by high-dose chemotherapy and autologous stem cell transplantation (ASCT). However, this approach is not curative, and residual disease leads to disease relapse. Sustaining response and postponing relapse following ASCT is an important clinical goal, as early progression is associated with an increased risk of death.
Lenalidomide (LEN) maintenance therapy has emerged as an important standard of care post-ASCT. Several clinical studies have shown that patients who received LEN maintenance therapy after ASCT had significantly longer progression-free survival (PFS) and overall survival (OS) in comparison with those who did not receive maintenance therapy. Other studies have indicated that active LEN maintenance treatment does not impair quality of life (Tay J, et al. Blood. 2017;130:abstract 2150). A recent EU5 cost-impact analysis suggested that LEN maintenance is potentially cost saving on direct medical costs by 24% over a 5-year period (Jackson G, et al. Blood. 2017;130:abstract 3405). To date, no study has specifically assessed the cost-effectiveness of LEN as maintenance treatment.
Aims: To assess the cost-effectiveness of LEN treatment versus no maintenance treatment in transplant-eligible NDMM patients from a Dutch healthcare service perspective.
Methods: A partitioned survival model structure was selected to provide a good fit to the supporting efficacy and safety data. The model was structured around 3 primary health states relevant to an NDMM patient's treatment trajectory: pre-progression state (encompassing on- and off-treatment periods), post-progression state (encompassing periods just prior to second-line treatment, on second-line treatment, and post-second-line treatment), and death.
Efficacy and safety data were taken from a pooled meta-analysis of the CALGB 100104, GIMEMA RV-MM-PI-209, and IFM 2005-02 studies. Parametric models were used to estimate long-term survival.
Utility data were applied from a real-world setting captured in the Connect® MM Disease Registry, which was used to calculate the progression-free (LEN), progression-free (no treatment), progressive disease (treatment-free), and progressive disease (second-line treatment) utilities. A 21 out of 28-day cycles dosing regimen for LEN was applied as recommended in the Dutch HOVON clinical guidelines. Costs (2016) and subsequent therapy data were derived from published literature and sources appropriate for the Dutch market. All drug costs are presented at list price. Healthcare resource utilization was informed from a EU5 (France, Germany, Italy, Spain, and the UK) real-world study (Ashcroft J, et al. Int J Hematol Oncol. 2018;Epub ahead of print).
The total costs, life years gained (LYG) and quality-adjusted life years (QALYs) were estimated over a lifetime horizon. Multiple scenario and sensitivity analyses were conducted to test the robustness of the model results to key assumptions and data inputs.
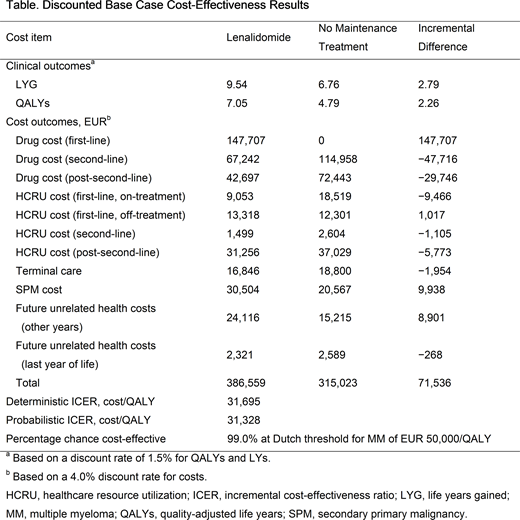
Results: The cost-effectiveness model predicted a QALY gain of 2.26 and a LYG of 2.79 for LEN in the base case analysis (Table). First-line drug costs of LEN contributed to an increase of EUR 147,707 in total costs versus no maintenance treatment. However, this was partially offset by savings of EUR 77,462 in subsequent treatment costs.
LEN was shown to represent a cost-effective use of resources when compared with the Dutch willingness-to-pay (WTP) threshold for NDMM of EUR 50,000/QALY.
Scenario analyses showed LEN remained cost-effective in settings representative of Dutch clinical practice. For instance, use of only the Dutch recommended dose (10 mg with dose reductions if needed) of LEN for NDMM gave an incremental cost-effectiveness ratio (ICER) of EUR 30,709. Scenario ICERs evaluating the key assumptions, aligning the subsequent therapy data as per in-trial use (EUR 49,059) and up to 83.9% of patients receiving 28 out of 28-day dosing, also remained below the WTP threshold.
Conclusions: Introducing LEN as a maintenance therapy post-ASCT delays progression, improves survival, and reduces subsequent treatment-line costs. The use of LEN post-ASCT is cost-effective in comparison with no maintenance therapy in the Netherlands.
Uyl-de Groot:Merck: Research Funding; Janssen- Cilag: Research Funding; Gilead: Research Funding; Genzyme: Research Funding; Celgene Corp.: Research Funding; Boehringer Ingelheim: Research Funding; Bayer: Research Funding; AstraZeneca: Research Funding; Astellas: Research Funding; Amgen: Research Funding; Roche: Research Funding; Sanofi: Research Funding. Ramsden:Celgene Corp.: Consultancy; BresMed: Employment. Boersma:Celgene BV: Employment, Equity Ownership. Zweegman:Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene Corp.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding. Dhanasiri:Celgene International: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal